0
There are not items in your cart.
Visit Shop
Product is not available in this quantity.
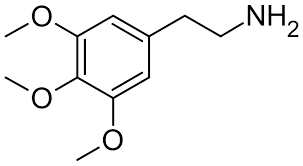

OVERVIEW:
Perform soxhlet extraction with 95% ethanol -> clean with diethyl ether and chloroform, combine and set aside organic fraciton containgin entourage of alkaliods _> concentrate then acidify ethanolic portion with sulfuric acid to 4-5pH -> slowly cool to form mescaline sulfate crystals
Starting material: Powdered dried peyote buttons (Lophophora williamsii/lewinii, preferably the green upper part — Heffter noted higher alkaloid content there).
Optional recovery from mother liquors (if yield low):
Characterization for your report:
This matches Heffter's emphasis on ethanol → ether partitioning → sulfate crystallization, without needing chloroform (though he used it later for exhaustive recovery of residues).
SOURCES
This is a google translated form of the quantative extraction (crude alkaloid + purified isolation) from Heffters original German paper (which may be found below this translation). Translation errors may be present, formatting errors are most certaibly present. The above guide was deduced from this translation. Anybody who can attest to this google translation, please reach out to JD.
To determine the alkaloid content, the following procedure was used: 50 g of the drug were extracted in a Soxhlet apparatus with 96% alcohol until the extract ran clear. The extract was freed from alcohol by evaporation, and the residue was taken up in water, whereby the above-mentioned wax and resin remained undissolved. I made the aqueous solution strongly alkaline with ammonia and extracted it three times with chloroform. The residue remaining after distilling off the chloroform was dried in a vacuum over sulfuric acid and weighed. It is calculated below as crude alkaloid. However, since, according to experience, small amounts of resin always pass into the chloroform, and since a titration of the strongly brown-colored solution was impracticable, the residue was dissolved in a small amount of warm water and carefully neutralized with sulfuric acid. The resin remained undissolved. The filtered solution of the alkaloid sulfates was evaporated and dried in a desiccator to constant weight. This mixture of sulfuric acid salts is a brown, largely crystalline mass.The following figures were obtained using the described methodAccording to the method described, the following figures were obtained:1. Mescal Buttons from E. Merck, alcohol extract . . . . 18.0% crude alkaloid . . . . . 5.8% = alkaloid sulfate . . . . 6.0%2. "Peyote" of the Huichol people (obtained from Mr. C. Lumholtz), alcohol extract . . . . 7.6%Crude alkaloid . . . . . 1.4% = alkaloid sulfate . . . . 1.5% - It should be noted that this "Peyote" consisted of chopped whole plants, while the "Mescal Buttons" represent only the upper, chlorophyll-containing part.3. Dried cacti, which had been freshly obtained from Saltillo.a) Upper, chlorophyll-containing part:Alcohol extract . . . . 11.9% crude alkaloid . . . . . 3.07% = alkaloid sulfate . . . . 3.6%b) Root pieces:Alcohol extract . . . . 5.5% crude alkaloid . . . . . 0.5% alkaloid sulfate . . . . 0.5% From these figures, the remarkable fact emerges that the alkaloids are primarily localized in the above-ground part of the plant. The highest figures of 6.0% and 3.6% are found in these samples.Furthermore, we observe that the alkaloid content in A. Lewinii is not as high as in A. Williamsii. The latter contained 3.5% free alkaloid in the dried state, while only 1.5% of the sulfate salts could be isolated from the former.The isolation of the alkaloids is carried out in principle exactly as described for the quantitative determination. However, it is advisable to precede the chloroform extraction with five repeated extractions with ether. This yields a large portion of the alkaloids relatively free from coloring impurities.The chloroform extraction is nevertheless necessary to recover the last traces of mescaline and anhalonidine.The treatment of the residues from both extracts is the same. The syrupy masses, freed from ether and chloroform, are mixed with a little hot water and precisely neutralized with sulfuric acid. Most of the substance dissolves, and the solution is filtered while hot. Beautiful, characteristic crystals of mescaline sulfate then separate out, either immediately upon cooling or after evaporation on a water bath. Further portions of crystals can be obtained by further concentration and later by adding alcohol; these are more strongly colored brown and represent a mixture of mescaline and anhalonidine sulfate. Finally, the mother liquor is evaporated to dryness in a vacuum and treated with absolute alcohol. If any further precipitation occurs at this point...the process is repeated until this is no longer the case. One can then be certain that no significant quantities of the two aforementioned alkaloids remain in the mother liquor. In this way, it is possible to completely process the chloroform extract down to a resinous residue, while a considerable, usually strongly colored mother liquor remains from the ethereal extract. This is first completely freed from alcohol on a water bath and later in a vacuum, and then taken up with plenty of water, whereby coloring substances usually remain undissolved. By carefully adding barium chloride solution, as long as a precipitate forms, the alkaloid sulfates present in the solution are converted into chlorides. After filtering off the barium sulfate, the solution is concentrated and left to stand in a vacuum. Then, after a shorter or longer period, sometimes only after the addition of alcohol, the needles of anhalonine hydrochloride crystallize out. If nothing more crystallizes out, even after further concentration and addition of alcohol, the mother liquor is diluted with water and...It is precipitated with a cold, aqueous sublimate solution. Previously, I used an alcoholic solution, but found that this resulted in losses, sometimes even preventing any precipitation at all. Upon the addition of mercuric chloride, which should not be added too sparingly, a brown, greasy precipitate immediately forms, adhering firmly to the walls of the beaker. If no further turbidity occurs upon continued addition, the supernatant liquid is poured into another vessel. After standing for some time, yellow or white crystalline warts separate out, which are collected on a filter. The initially obtained brown precipitate is treated separately with a large quantity of boiling water, in which it largely dissolves. The brown solution is decolorized with animal charcoal. Upon cooling, droplets separate from the filtrate, which solidify into yellowish crystalline spheres. These represent the mercury double salt of lophophorine.By concentrating the mother liquors of these crystals, further crystal deposits can be obtained, consisting of mercury compounds of anhalonidine and perhaps one or more other alkaloids, about which I cannot yet provide any further details, as they have so far only been obtained in very small quantities and could not be separated. The crystals mentioned above, which separate out only after prolonged standing, are also such mercury double salts.Before I proceed to the description of the individual alkaloids, the separation method of mescaline and anhalonidine must first be discussed briefly. This separation requires considerable patience and care if one is to avoid significant losses. The first two crystal precipitates usually consist of pure mescaline sulfate; it can be obtained in the purest form immediately by recrystallization from water or, better, from boiling methyl alcohol.When the later crystal fractions are treated with hot methyl alcohol, it is observed that they dissolve only partially. A white, crystalline powder remains undissolved. Upon cooling, pure mescaline sulfate first separates from the solution, followed by indistinct crystals of a mixture of sulfates of both alkaloids. These, as well as the residue insoluble in methyl alcohol, which also still contains mescaline, are dissolved together in water and converted into the chlorides by careful addition of barium chloride. From the filtrate of barium sulfate, which is concentrated on a water bath, the mostly slightly pink-colored, clear prisms of the anhydrous chloride separate after standing for several days in a desiccator.lonidine hydrochloride precipitates. If a large amount of material has been processed, mescaline sulfate can be obtained again from the mother liquor by further reaction with silver sulfate, and so on. It is absolutely essential to check the purity of the crystalline fractions, especially of anhalonidine, under the microscope. The uniformity of the salt in question can be particularly easily verified by adding some platinum chloride to a few drops of the aqueous solution: the resulting chloroplatinate crystals are so characteristic and so sparingly soluble that even very small amounts of mescaline or anhalonidine can be detected under the microscope.
https://www.sciencedirect.com/science/article/abs/pii/S0022354915389474
https://sci-hub.ru/10.1007/BF01825267
https://drive.google.com/file/d/1q1_Vc_bj9N6u08a-8zTpng3jX6YFSGZM/view?usp=sharing
